Fetal Bovine Serum (FBS)
Overview
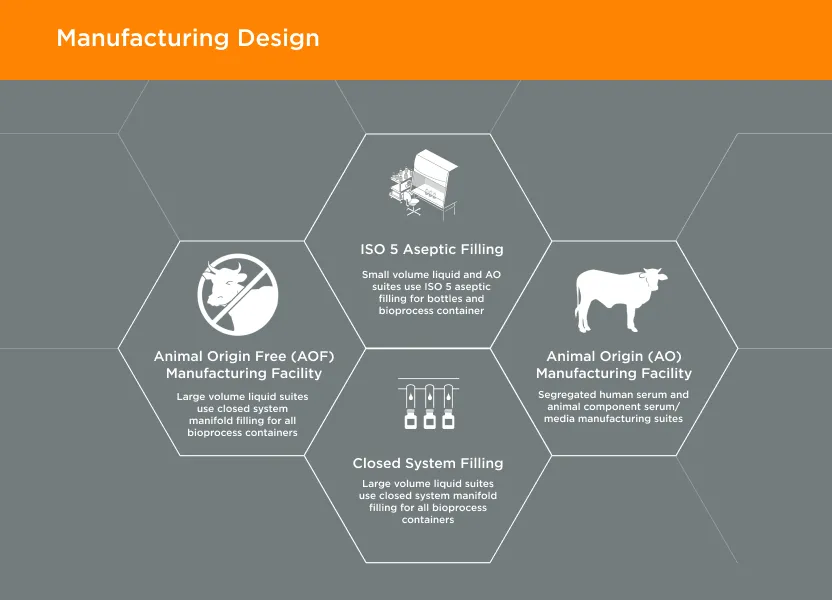
GeminiBio offers a wide range of FBS quality options – manufactured under cGMP – with complimentary samples, lot reservation, heat inactivation, and cold storage to improve our customer’s workflow productivity, reproducibility, and speed. Our product portfolio spans US and USDA sourced FBS, as well as specialty FBS products such as stem cell qualified, insect cell qualified, dialyzed, as well as other options.