GeminiBio has acquired the manufacturing and global sales rights for Bio-Techne’s R&D Systems FBS Brands, including Optima, Premium Select, and Premium


For over 35 years, GeminiBio’s cell culture products have provided customers – from research through production – with the tools needed to improve their workflow productivity, reproducibility, and speed.
Overview
The cell culture workflow – prep, modify, and expand – is often challenged by costly rework and inadequate cell growth, leading to a significant strain on resources and timelines. To help optimize cell culture workflow, GeminiBio offers a comprehensive line of fetal bovine serum (FBS), human serum (HSAB), and other animal sera, cell culture media, cell culture supplements and reagents, growth factors and cytokines, as well as lab equipment, consumables and reagents.
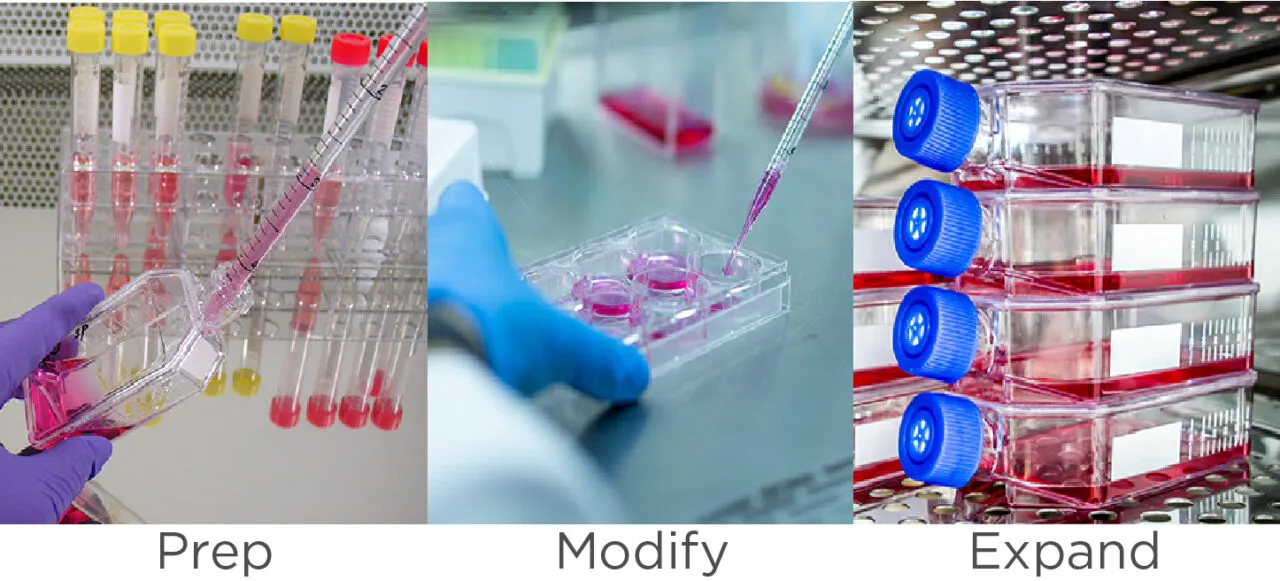

GeminiBio enables researchers to optimize their cell culture workflow to improve productivity, reproducibility, and speed. Our product portfolio includes a comprehensive line of fetal bovine serum (FBS), human serum (HSAB), other animal serums, cell culture media, supplements and reagents, growth factors and cytokines, plus lab consumables.

GeminiBio enables biopharma customers to optimize their upstream and downstream workflows to improve productivity and speed. Our product portfolio includes custom cGMP manufactured media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, and a comprehensive line of sera, including fetal bovine serum (FBS), as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables Gene Therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables Cell Therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing, as well as therapeutic cell manufacturing, by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters. GeminiBio supports therapeutic cell manufacturing with a comprehensive line of human serum products, and mesenchymal stem cell (MSC) and T-Cell media.

GeminiBio enables Life Science Tools Manufacturers to significantly expand the scale – and flexibility – of their cGMP process liquid manufacturing, allowing them to better meet demanding end-customer requirements. Through our cGMP process liquid contract (private label) manufacturing capabilities, which include cGMP batch sizes from 10-liters to 10,000-liters, and ability to fill into diverse container types, and sizes ranging from 500 mL bottles to 1,000-liter pallet tanks, life science tools manufacturers can optimize their market penetration and end-customer satisfaction.

GeminiBio enables researchers to optimize their cell culture workflow to improve productivity, reproducibility, and speed. Our product portfolio includes a comprehensive line of fetal bovine serum (FBS), human serum (HSAB), other animal serums, cell culture media, supplements and reagents, growth factors and cytokines, plus lab consumables.

GeminiBio enables biopharma customers to optimize their upstream and downstream workflows to improve productivity and speed. Our product portfolio includes custom cGMP manufactured media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, and a comprehensive line of sera, including fetal bovine serum (FBS), as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables Gene Therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters, as well as “off the shelf” water for injection (WFI) quality water.

GeminiBio enables Cell Therapy customers to optimize their upstream and downstream workflows to improve productivity and speed. We support the unique needs of plasmid DNA and viral vector manufacturing, as well as therapeutic cell manufacturing, by providing custom manufactured cGMP media, buffers, and other process liquids in batch sizes from 10-liters to 10,000-liters. GeminiBio supports therapeutic cell manufacturing with a comprehensive line of human serum products, and mesenchymal stem cell (MSC) and T-Cell media.

GeminiBio enables Life Science Tools Manufacturers to significantly expand the scale – and flexibility – of their cGMP process liquid manufacturing, allowing them to better meet demanding end-customer requirements. Through our cGMP process liquid contract (private label) manufacturing capabilities, which include cGMP batch sizes from 10-liters to 10,000-liters, and ability to fill into diverse container types, and sizes ranging from 500 mL bottles to 1,000-liter pallet tanks, life science tools manufacturers can optimize their market penetration and end-customer satisfaction.
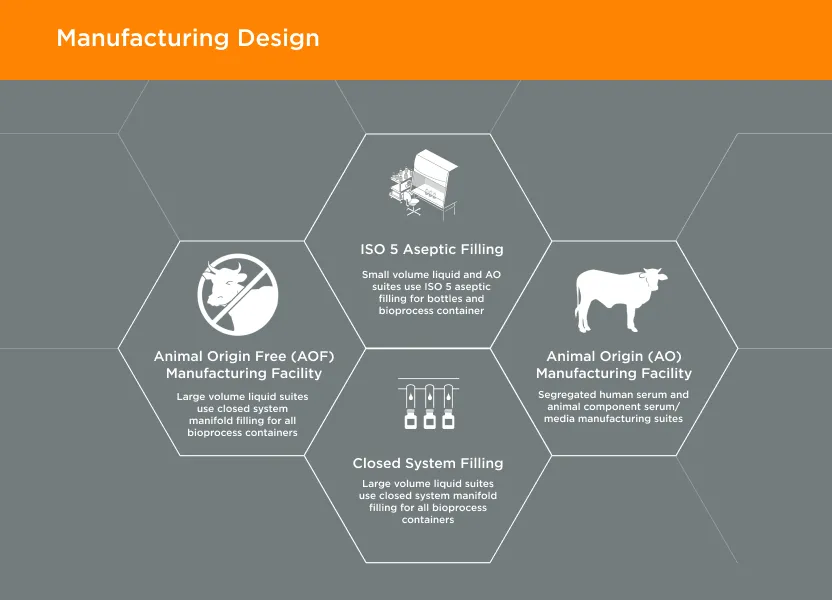
GeminiBio’s facilities are designed with current Good Manufacturing Practices (cGMP) and our customer’s quality requirements in mind. Our animal origin free (AOF) manufacturing facility is 1/4 mile away from our animal origin (AO) manufacturing facility. In addition, our cGMP warehouse is designed with flows for both personnel and materials, including segregation of AO and AOF materials.


920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America

920 Stillwater Rd Suite 130, West Sacramento, CA 95605,
United States of America
