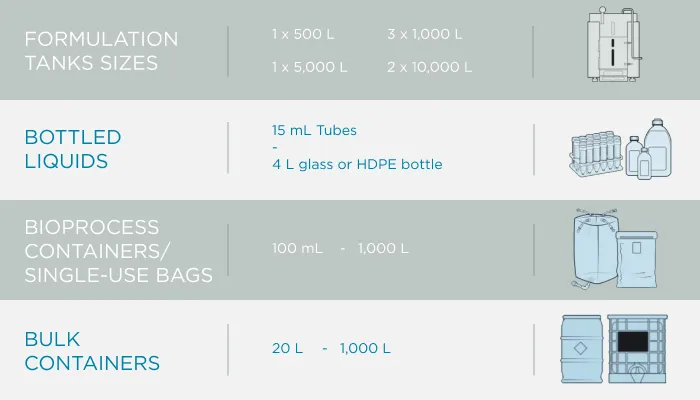
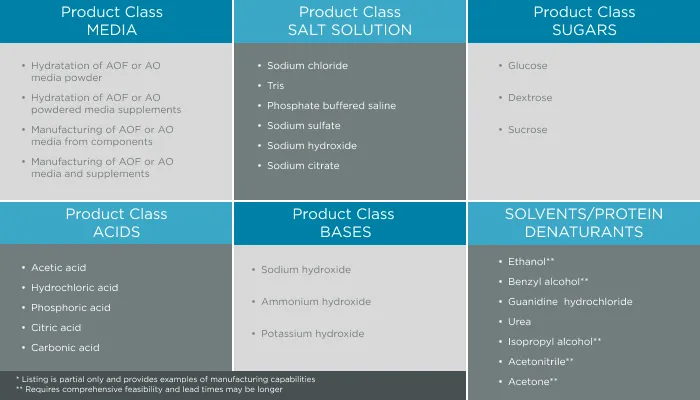
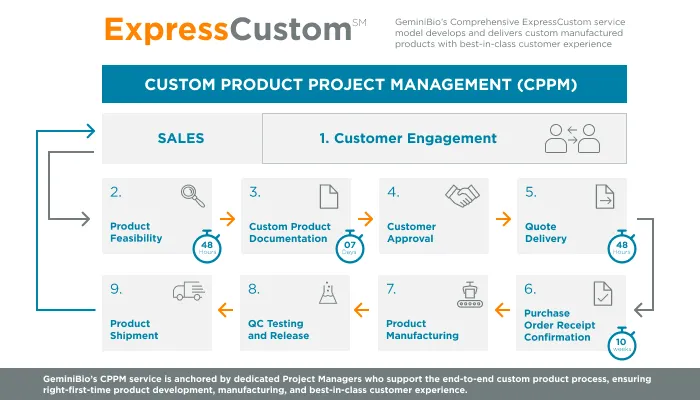
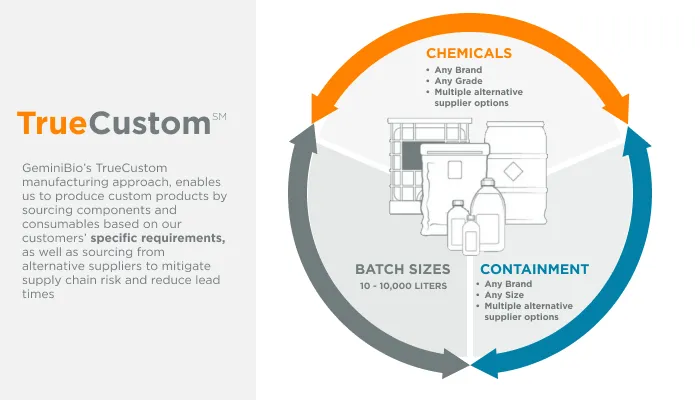
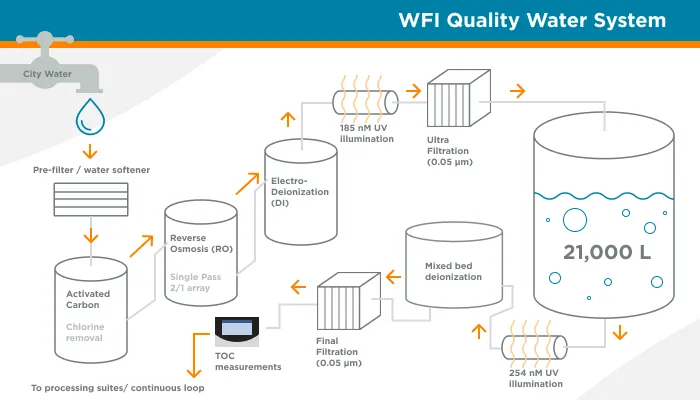
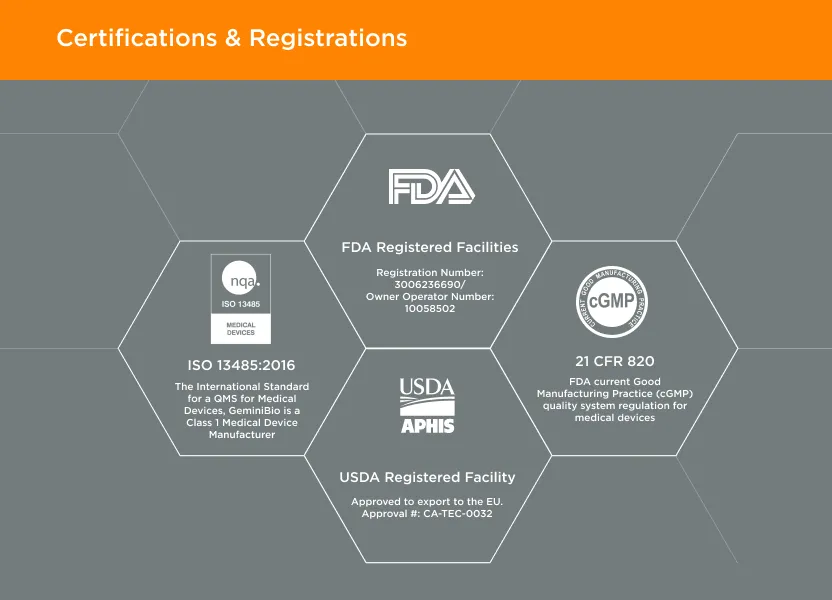
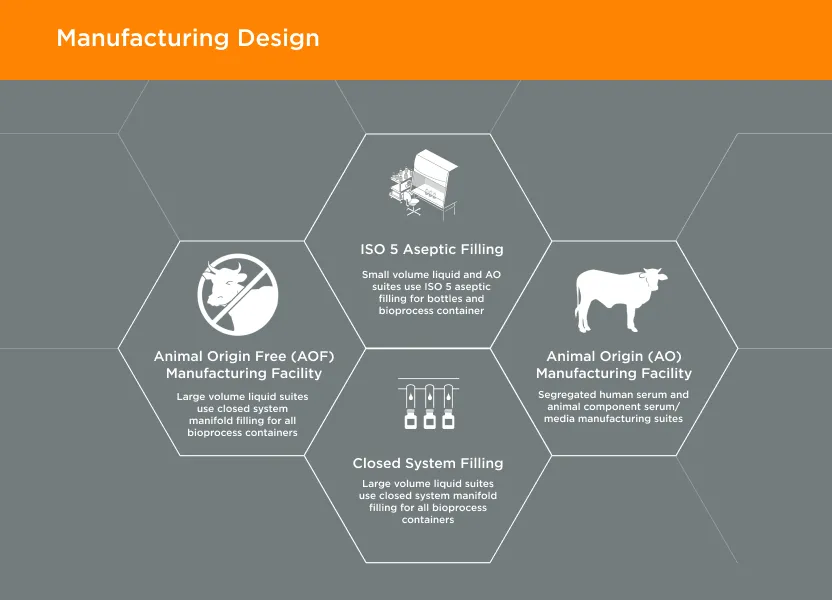
GeminiBio is uniquely positioned to help customers scale their process liquids. From RUO to cGMP products, preclinical to clinical scale cGMP batch sizes, and ultimately to commercial production scale cGMP batch sizes, GeminiBio has the capabilities required. In addition to custom manufacturing, GeminiBio offers a broad portfolio of off the shelf high purity water, including water for injection quality water, water for irrigation quality water, purified water, as well as cell and molecular grade water.